The transportation of dangerous goods is an important matter; from the identification of the substance to the packaging used and the length of transportation.
Limitations
Due to the nature of the packaging, some countries (states) or Airlines (operators) may have restrictions on quantities or may even refuse to accept packages containing infectious substances – be sure to check with the relevant dangerous goods regulations (IATA, ADR, IMDG) before you attempt to transport your goods – it’ll save you time and, quite possibly, money.
Classification
Infectious substances are classified as Class 6.2 (substances which are known or are reasonably expected to contain pathogens, which can cause disease in humans or animals), biological products, cultures, patient specimens and medical or clinical wastes.
Within the 6.2 classification, substances can be categorised within Category A or Category B.
Category A includes substances that are “capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy human or animals.”
Category B substances include any substances not meeting the criteria for inclusion for Category A.
Examples of Class A infectious substances:
- Affecting humans Affecting animals
- Coxiella burnetii (cultures only)
- Crimean-Congo hemorrhagic fever virus
- Dengue virus (cultures only)
- Eastern equine encephalitis virus (cultures only)
- Escherichia coli, verotoxigenic (cultures only)
- Ebola virus
- Flexal virus
- Francisella tularensis (cultures only)
- Guanarito virus
- African swine fever virus (cultures only)
- Avian paramyxovirus Type 1 – Velogenic Newcastle disease virus (cultures only)
- Classical swine fever virus (cultures only)
- Foot and mouth disease virus (cultures only)
- Lumpy skin disease virus (cultures only)
- Mycoplasma mycoides – Contagious bovine pleuropneumonia (cultures only)
- Peste des petits ruminants virus (cultures only)
Examples of Category B substances Includes:
- COVID 19 – Corona Virus
A level of professional judgement is required when determining whether a substance has a minimum likelihood that pathogens are present. This judgement relies on having knowledge of individual human or animal history, circumstances and endemic local conditions, enabling an accurate judgement to be made.
Identification
In order to fully understand how infectious substances should be packaged and labelled, it is important to identify the exact substance that will be transported – this requires knowing the ‘Proper Shipping Name’ – the name given to identify the hazard classification and composition of a dangerous substance, or the UN number associated with each particular type of infectious substance; UN2814, UN2900, UN3291 or UN3373.
By identifying the Proper Shipping Name, a shipper can find the appropriate Packing Instruction, which will identify the appropriate method of transportation, as well as any limitations, for example, the maximum capacity of infectious substances that can be shipped by air at any time is 4 kg.
Packing Infectious Goods
Packaging used in the transportation of infectious substances should follow Packing Instructions 620, 622 or 650, and comprise three components;
- A leak-proof primary receptacle (i.e. a test tube)
- Leak proof secondary packaging (i.e. larger plastic packaging, e.g Biobag for Cat B or Rigid plastic tube for Cat A)
- Outer packaging that has adequate strength for the capacity, mass and intended use, where:
- The smallest external dimension must be no less than 100mm (Packing instruction 620), or
- At least one surface of the outer packaging must have a minimum dimension of 100mm x 100mm (Packing instruction 650)
The primary receptacle or the secondary packaging must be capable of withstanding, without leaking, an internal pressure of 95 kpa or more, as well as temperatures between -40 and +55 degrees centigrade.
Where liquids are used, absorbent material must be used with capacity to absorb 100% of the liquid.
Packing Specifications and Performance Tests for Infectious Substances
For category A substances, packaging must be UN approved and will be subject to such tests as follows:
- Drop tests must be performed from a height of 9m (packages that are intended to contain dry ice must withstand an additional drop test)
- A puncture test must be carried out for packagings with a gross weight of more than 7 kg.
For Category B substances, packaging does not have to be UN approved; however must meet the criteria for Packing Instruction 650 and be subject to various tests, including a 1.2m drop test.
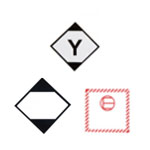
Infectious Goods Marking & labelling
The way outer packaging for infectious substances must be labelled is incredibly important – failure to apply the correct hazard labels, in-line with UN dangerous goods regulations, will result in items not being accepted for transport – the consequences, of which, could be catastrophic – particularly where substances require temperature control.
Before the transportation of goods, each package should be correctly labelled, to show:
- The shipper and consignee details
- The UN number and proper shipping name of each substance within the package
- A primary hazard label (and – where necessary – a label to identify a secondary hazard class)
- Markings to identify that the package should be kept one-way up (where liquids are present)
- The name and contact phone number for a responsible professional who can be contacted for more information about the package, e.g. a qualified doctor.
Approved Packaging
Here at Air Sea Containers, we have a range of infectious goods packaging solutions for both Category A and Category B (UN3373) substances. Unsure about which packaging you require? Contact us.
Information correct at time of publishing, 17th February 2017
 UK
UK