The transportation of dangerous goods is an important matter; from the identification of the substance to the packaging used for a compliant shipment.
The below article explains the process behind the transportation of infectious substances.
For this particular article we refer to IATA regulations as our source, but be sure to check the relevant transport regulations before you attempt to transport your goods, it’ll save you time and quite possibly money.
Classification of Infectious Substances
Infectious substances are classified as Class 6.2 (substances which are known or are reasonably expected to contain pathogens, which can cause disease in humans or animals), biological products, cultures, patient specimens and medical or clinical wastes.
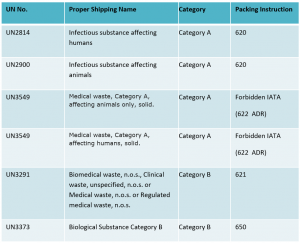
Infectious substances must be classified in Division 6.2 and assigned to UN2814, UN2900, UN3291, UN3373 or UN3549 as appropriate.
Within the 6.2 classification, infectious substances can be categorised within Category A or Category B.
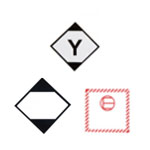
Examples of Class A infectious substances
Category A includes substances that are “capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals”.
Affecting humans UN2814 |
Affecting animals UN2900 |
|
|
There has been a lot of media coverage for Monkeypox recently, as a result ADR have clarified how to safely ship Monkeypox via road.
ADR Regulations – Under Multilateral Agreement M347 Under section 1.5.1 of ADR on the carriage of monkeypox virus the regulations are as follows:
- By derogation of paragraph 2.2.62.1.4.1, section 3.2.1. (Table A, Dangerous Goods List) and Chapter 4.1 of ADR, infectious substances containing monkeypox virus except for cultures of monkeypox virus may be carried under UN 3373 or UN 3291, as appropriate.
- The consignor shall include the following entry in the transport document, “Carriage in accordance with Multilateral Agreement M347”.
- This agreement shall be valid until 31 December 2025 for carriage on the territories of those ADR Contracting Parties signatory to this Agreement. If it is revoked before that date by one of the signatories, it shall remain valid until the above-mentioned date only for carriage on the territories of those ADR Contracting Parties signatory to this Agreement, which have not revoked it.
It is worth checking the relevant regulations for new updates especially when dealing with emerging pandemics.
Examples of Class B infectious substances
Category B consists of “infectious substance[s] which does not meet the criteria for inclusion in Category A”. This may include blood, tissue, excreta, secreta, and other fluids.
Identification of infectious substances
In order to fully understand how a substance should be packaged and labelled, it is important to identify the exact substance that will be transported – this requires knowing the ‘Proper Shipping Name’ – the name given to identify the hazard classification and composition of a dangerous substance, or the UN number associated with each particular type of infectious substance.
By identifying the Proper Shipping Name, a shipper can find the appropriate Packing Instruction, which will stipulate the appropriate packaging requirements, as well as any limitations or special provisions. To fully understand the regulations for your specific shipment(s), you must refer to the relevant regulation(s) for your mode of transport as these can differ. For example, IATA states UN3549 is forbidden by air but ADR states it is permitted by road.
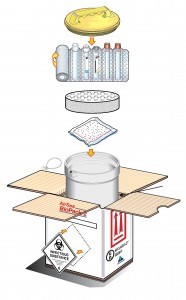
Packing infectious substances
Packaging used in the transportation of infectious substances via air should follow the relevant Packing Instructions – 621, 620, or 650.
PI 621 requires:
A rigid Outer packaging – for example – Drums, Jerricans & Boxes, meeting Packing Group II performance standards.
Category A and B infectious substances that require triple packaging
PI 620 and 650 comprise of three components;
- A leak-proof primary receptacle(s)
- Leak proof secondary packaging (Siftproof for solids)
- A rigid outer packaging that has adequate strength for the capacity, mass and intended use, where:
- The smallest external dimension must be no less than 100mm (PI 620), or
- At least one surface of the outer packaging must have a minimum dimension of 100mm x 100mm (PI 650)
The primary receptacle or the secondary packaging must be capable of withstanding, without leaking, an internal pressure of 95 kPa or more, *as well as temperatures between -40 and +55 degrees centigrade (*PI 620).
Where liquids are used, absorbent material must be used with the capacity to absorb 100% of the liquid.
Shippers responsibility
It is the shipper’s responsibility to ensure that they pack their substance/article correctly within the packaging. It is essential that the shipper packs the substance exactly as per the assembly sheet instructions, to ensure the chosen packaging performs to the standard it did during approval testing. If a shipper fails to pack the Dangerous Goods as per the assembly instructions, they risk invalidating the test certificate and compromising the capability of the packaging.
Infectious substance packaging specifications and performance tests
For category A substances UN2814 & UN2900, packaging must be UN approved and will be subject to a number of tests such as:
- Drop tests performed from a height of 9m (packages that are intended to contain dry ice must withstand an additional drop test)
- Puncture tests.
For Category B substances UN3373, packaging does not have to be UN approved; however, must meet the criteria for PI 650 and be subject to various tests, including a 1.2m drop test.
Infectious substance shipping marking and labelling
The way outer packaging for infectious substances must be labelled is incredibly important – failure to label adequately, in-line with UN regulations, will result in items not being accepted for transport.
Before the transportation of goods, each package should be correctly labelled, shippers must refer to the regulations for exact marking and labelling requirements – examples of such are:
- The shipper and consignee detail
- The proper shipping name and UN number of each substance within the package
- Hazard class label
- Markings to identify that the package should be kept one-way up (UN2814 & UN2900, where liquids are present)
- The name and contact phone number of a person responsible (PI 650 either on package or air waybill)


Limitations on infectious substance packaging
When sending shipments by air, some countries (states) or Airlines (operators) may have restrictions on quantities or may even refuse to accept packages containing infectious substances. For instance, IATA states ‘Infectious substances other than human blood products, human urine and human tissue, are prohibited from entry to Australia without prior approval from Australian Health Authorities. Be sure to check the state and operator variations on the Packaging Instruction to see all relevant limitations.
Infectious substance packaging solutions at Air Sea Containers
There are many regulatory factors to consider when shipping infectious substances and numerous packaging options available.
Air Sea Containers has a range of infectious substance packaging solutions for both Category A and Category B substances, if you would like advice on what packaging you require you can contact us at UK@airseadg.com
 UK
UK