The safe and compliant transport of infectious substances is critical to protecting human and animal health. Among these substances, UN 3373 refers specifically to Biological Substance, Category B, a classification under Class 6.2 Infectious Substances. Unlike Category A substances, which pose a high risk causing permanent disability, life threatening or fatal disease in otherwise healthy humans or animals, Category B includes substances that are not capable of causing life-threatening or fatal diseases during transport but still require strict handling and packaging protocols in line with UN dangerous goods transport regulations.
Understanding UN 3373
UN 3373 applies to infectious substances that do not meet the criteria for Category A. These are typically clinical samples or diagnostic specimens that may contain pathogens but are not identified as Category A substances. Even though they pose a lower risk, improper handling can still result in exposure or contamination, making correct packaging essential.
Why Is Compliant Packaging for UN 3373 Important?
Infectious substance packaging plays a vital role in preventing leakage and accidental exposure during transport. If the primary receptacle fails, the packaging must contain the substance and protect anyone who comes into contact with the shipment, including couriers, lab workers, and customs agents. Packaging for UN 3373 ensures that the biological substance is securely enclosed, even if subjected to rough handling or environmental conditions during shipping.
Packaging Requirements for UN 3373
Shipments classified under UN 3373 must adhere to Packing Instruction P650, which outlines the packaging requirements for C ategory B infectious substances. These requirements include the triple packaging system, designed to ensure containment and prevent leakage:
ategory B infectious substances. These requirements include the triple packaging system, designed to ensure containment and prevent leakage:
- Primary Receptacle
- Holds the actual specimen or substance
- Must be leakproof
- If there are multiple primary receptacles, each must be individually wrapped or cushioned
- Secondary Packaging
- Also, leakproof either the primary of secondary receptacles must meet 95kPa pressure test
- Must contain enough absorbent material to absorb the entire contents of the primary receptacle in case of breakage or leakage
- Outer Packaging
- Must be rigid, but does not require UN approval (unlike Category A packaging)
- Must be clearly marked with the UN 3373 diamond-shaped label and “Biological Substance, Category B”
- Packaging for Category B does not have to be UN approved, however, it does need to have demonstrated that it can pass certain performance tests.
Unlike packaging for Category A, UN 3373 compliant packaging does not require UN certification (carry a UN specification mark) but must still demonstrate the ability to withstand performance testing, such as 1.2 Metre drop tests and pressure tests where required. It’s critical that the packaging is assembled exactly as instructed by the manufacturer to ensure compliance and safety.
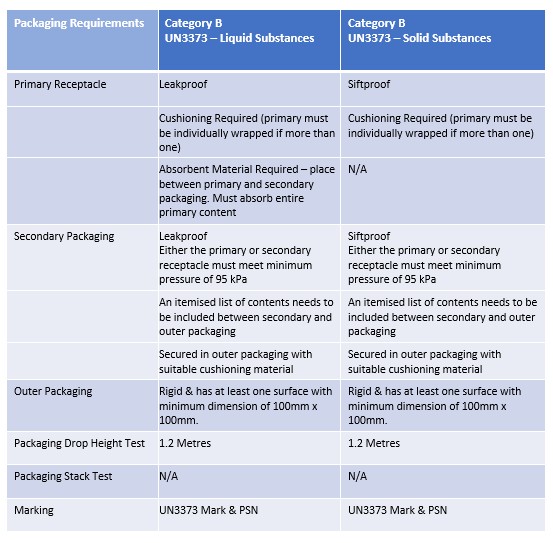
This table summarises the packaging requirements in P650.
Shippers must refer to P650 for full provisions.
Additional Considerations
- Category B substances may be shipped in UN approved Category A packaging, though this is not required.
- Conversely, Category A substances must never be transported in Category B packaging.
- Always confirm with your packaging supplier that the packaging used meets the relevant testing standards for UN 3373 shipments.
How to ensure you assembly correctly and compliantly
At Air Sea USA we provide assembly instructions for all our combination packaging, to help shippers assemble the packaging correctly and compliantly, you can find our assembly instructions for our postal pack and larger bioshipper 1 here. if your UN 3373 substance is temperature sensitive you can use our temperature control packaging specifically made to carry infectious substances.

Conclusion
Transporting biological substances under UN 3373 comes with specific packaging requirements. By following Packing Instruction P650 and ensuring that triple-layer packaging is used and assembled correctly, shippers can minimize risks and maintain regulatory compliance. While the requirements are less stringent than those for Category A substances, the importance of safety, accuracy, and adherence to instructions cannot be overstated.
If you are unsure about which packaging is appropriate for your Cat B biological samples, contact us we are happy to help.
Interested in shipping Category A substances read our how to ship infectious substances article here.
Information correct at the time of publication 20/06/25
 US
US